Abstract
Introduction:
Chimeric antigen receptor T cell therapy (CAR-T) is a novel treatment that utilizes T cells by augmenting them using vector viruses to add antigens to target cancer cells. In 2017, FDA approved CD-19 CAR-T for relapsed/refractory diffuse large B-cell lymphoma and acute lymphoblastic leukemia patients ≤ 26yr old. Unique toxicities associated with CAR-T therapy include cytokine release syndrome (CRS) and immune effector cell-related neurotoxicity (ICANS). Lower-grade CRS and ICANS are managed with tocilizumab, an interleukin-6 antagonist, and steroids. Management of higher-grade CRS and ICANS requires intensive care unit (ICU) admission. Our understanding and management of CRS and ICANS continue to evolve. In this analysis, we conducted a retrospective review using the Vizient database® to investigate toxicity incidence and resource utilization among patients admitted for CAR-T therapy between 2017 and 2020.
Methods:
We used The Vizient® CDB database to analyze admissions for CAR-T infusion for patients over 18 years of age receiving FDA approved CD19 CAR-T axicabtagene ciloleucel (axi-cel) and tisangenlecleucel (tisa-cel) between 2017 to 2020. We compared patients who received CAR-T between October 2017 and March 2018 (group 1) to those who received CAR-T therapy between October 2019 and March 2020 (group 2). Due to the lack of diagnosis code for CRS or ICANS until 2021, surrogates billing codes such as fever, sepsis, dyspnea were used for CRS. In regards to ICANS, we used codes for febrile seizure, febrile convulsions, altered mental status, somnolence, and stupor. In addition, other adverse events such as weakness and nausea were also collected.
Results:
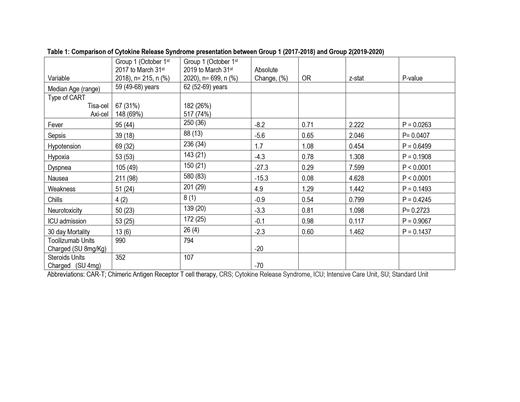
Eighty-one institutions had performed CAR-T in the period 2017 through 2020. The 2017-2018 period (group 1) included 215 patients, with a median age of 59 (49-68) years, while the CAR-T recipients in 2019-2020 (group 2) had 655 patients with a median age of 62 (52-69) years. Tisa-cel and Axi-cel was administered to 31% (n= 67) and 69% (n= 148) in group 1 and 26% (182) and 74% (n= 517) of group 2 patients respectively. The incidence of sepsis in group 1 was 18% vs. 13% in group 2, with an absolute difference of -5.8% (P value=0.04). Fever and dyspnea were the most common presentations of CRS present in 44.2% and 49% in group 1 and 35% and 28% in group 2, respectively. The incidence of fever decreased by 8.2% (p=0.02) in group 2 compared to group 1. The incidence of hypoxia was 24.7% vs. 20.5%, and the incidence of hypotension was 32.1% and 33.8% in groups 1 and 2, with no statistically significant difference between the two groups (p=0.64 and 0.19). The incidence of neurotoxicity decreased slightly in group 2 compared to group 1, but it was not statistically significant (P= 2723). Overall ICU utilization was 24.7 and 24.6% in both groups (p=0.9). The 30 days mortality in groups 1 and 2 was 6% vs. 3.7%. Tocilizumab utilization decreased by 20%, and dexamethasone or equivalent steroid usage decreased by 70% in group 2 compared to group 1. (Table 1)
Conclusions:
The incidence of CRS and ICANS among recipients of CAR-T remains high, with up to one-fourth of the patients requiring ICU, which has remained static. However, the general use of tocilizumab and steroids has decreased by 20% and 70%, respectively, possibly due to the implementation of consensus grading and operation protocols that may have increased awareness and judicious early interventions.
Mahmoudjafari: Incyte: Membership on an entity's Board of Directors or advisory committees; Omeros: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal